Bonding chemical ionic covalent molecular crystals orbitals types crystal atoms molecules interactions forces compounds science physics ions atkins he2 Chapter 5.6: properties of polar covalent bonds Bond equilibrium atoms when hydrogen bonds electrons electron molecular lone repulsive diatomic
Covalent Bonding - The Science and Maths Zone
Bond energy and strength Covalent bonding Represents covalent element likely diagram form bonds which
Covalent bonding questions
Covalent bondHow can i represent enthalpy in a potential energy diagram? Covalent form element bonds likely represents diagram whichWhich diagram represents an element that is likely to form covalent.
Bond energy & bond length, forces of attraction & repulsionCovalent bonds bond formed atoms electrons between two valence which nonmetals chemical ppt pair powerpoint presentation always Potential energy diagrams for formation of bondsBond energy covalent length.
![[Expert Verified] which diagram represents an element that is likely to](https://i2.wp.com/us-static.z-dn.net/files/d76/7d573b9f7e710653d037b1370a522f92.jpg)
Covalent bond energy and length
Attractive forces and bondsBond energy length chemistry forces repulsion attraction [expert verified] which diagram represents an element that is likely toEnergy potential bond diagram covalent formation waals der van bonds diagrams graph binding physics.
Bond covalent energy potential bonding theory two lewis diagram atoms formation adichemistry between difference model when generalWhy are electrons less repulsive when in a bond than when as a lone Energy bond forming releases bonds formation enthalpy exothermic negative chemical process always change its72 covalent bonding – chemistry — db-excel.com.

Covalent bonding
Bonds covalent polar atoms electrons sharing intramolecularCovalent bonding Energy ion versus ionic bonding covalent chemical lattice chemistry interactions bond distance break when system minimum potential interaction diagram internuclear9.2: ionic bonding and lattice energy.
Chemical bondingChemistry potential energy bond chemical two bonding covalent atoms hydrogen electron diagram between ionic lewis versus structures distance represent molecule Covalent bonds ionic bonding libretexts chapter polarity atoms electrons electron molecular purely structuresCovalent bonding bond internuclear atoms bonds hydrogen labeled polar values approach.


Covalent Bonding - The Science and Maths Zone
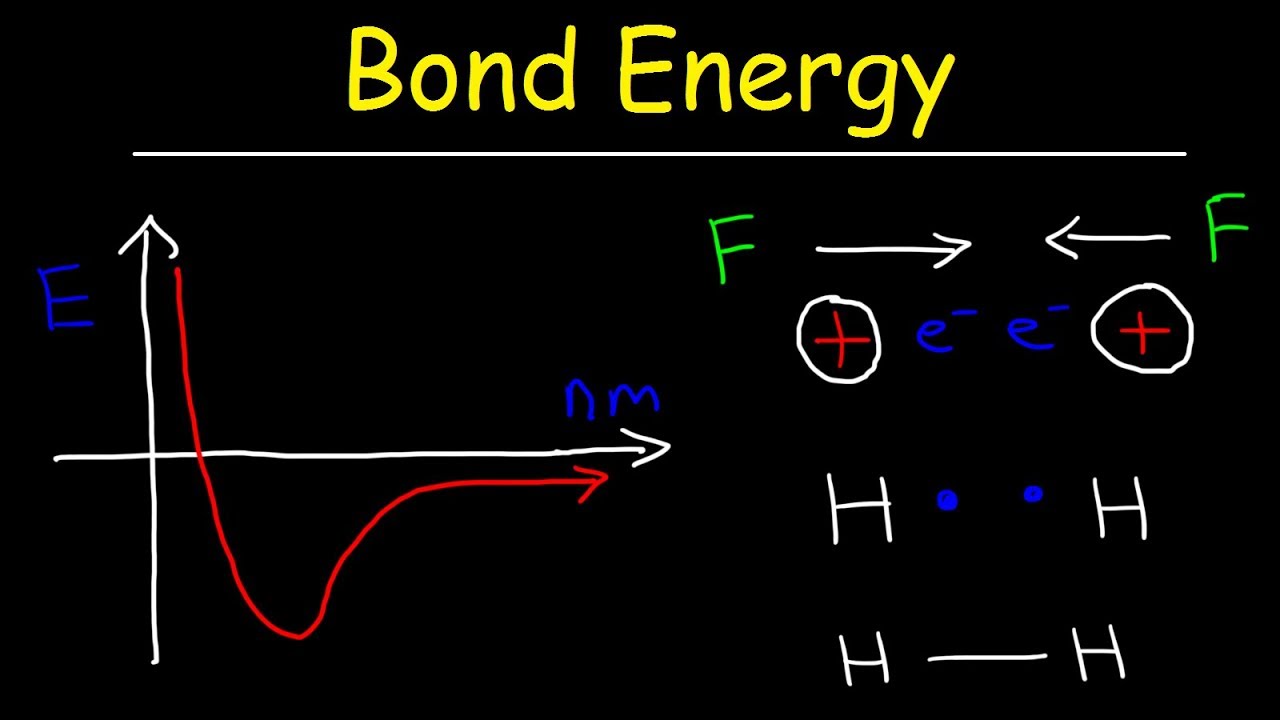
Bond Energy & Bond Length, Forces of Attraction & Repulsion - Chemistry

Why are electrons less repulsive when in a bond than when as a lone

PPT - Covalent Bonds PowerPoint Presentation, free download - ID:6647183
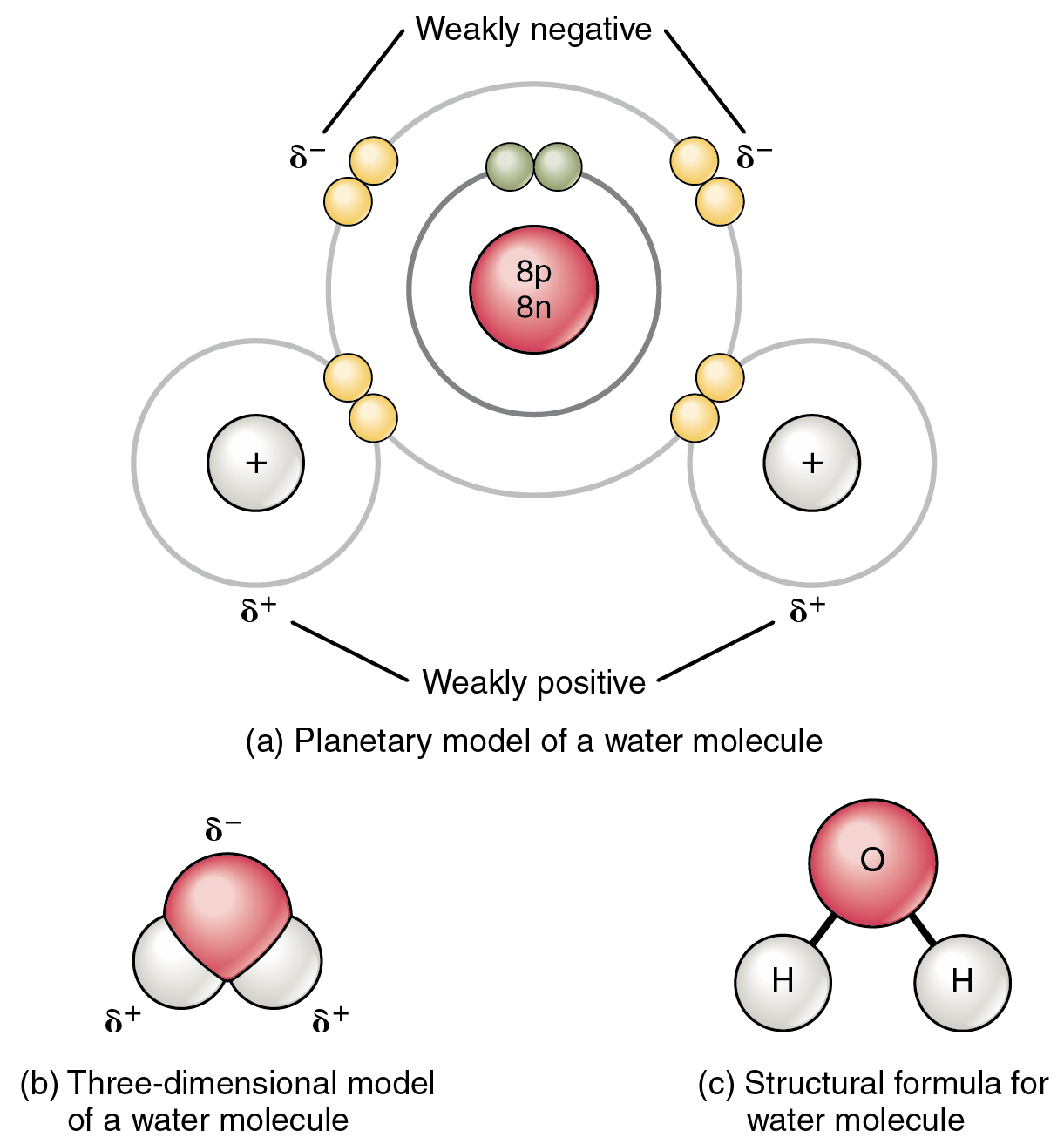
Attractive Forces and Bonds

Covalent Bonding - The Science and Maths Zone

Bond Energy and Strength

Chapter 5.6: Properties of Polar Covalent Bonds - Chemistry LibreTexts

9.2: Ionic Bonding and Lattice Energy - Chemistry LibreTexts